1 of 1
Click on image to open expanded view
Item No.
49897
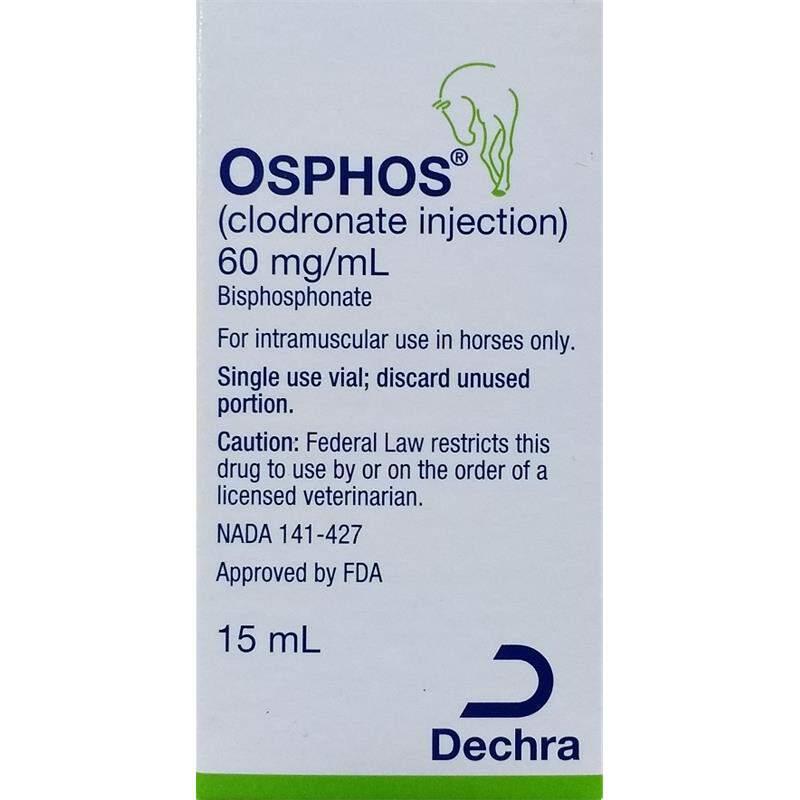
Osphos Inj 60mg/mL, 15 ml Rx
AutoShip & Save 20%
- Save on your Autoship
- Hassle-free: Easily Skip or Change Shipment Dates.
- Commitment-free: Cancel anytime
- Free Shipping.
With AutoShip, we remember so you don’t have to! Learn more
Osphos Inj 60mg/mL, 15 ml Description
Osphos Injection contains clodronate to help horses with issues caused by navicular syndrome. It aids in symptom relief, slowing the disease's progression. The ready-to-use, easy-to-use vial does not require dilution.
What is Osphos Inj 60mg/mL, 15 ml?
OSPHOS is an injectable bisphosphonate solution for the control of clinical signs associated with navicular syndrome in horses four years and older. OSPHOS inhibits bone resorption by binding to calcium phosphate crystals (inhibiting their formation and dissolution), and by exerting direct cellular effects on osteoclasts. OSPHOS is supplied as 15 mL (900 mg) of clodronate disodium (60 mg/mL) per vial and is ready-to-use (no reconstitution or dilution required).
Which animals/pets is Osphos Inj 60mg/mL, 15 ml for?
For use in horses ages 4 and up.
Osphos Inj 60mg/mL, 15 ml uses
Features of Osphos:
- Prevents bone resorption because it exerts direct effects on the cells of osteoclasts and binds to calcium phosphate crystals, which inhibits their dissolution and formation
- Excellent option for horses proven through radiography to have bony changes caused by navicular syndrome
- Injectable solution of bisphosphonate that aids in the control of the clinical signs of navicular syndrome
- The only option injected intramuscularly
- Contains 60mg clodronate in each mL
What does Osphos Inj 60mg/mL, 15 ml do?
Osphos works by inhibiting resorption of bone. It exerts direct effects on the cells of osteoclasts and binds to calcium phosphate crystals. This inhibits their dissolution and formation.
Osphos Inj 60mg/mL, 15 ml side effects
Field studies have shown that the most frequently-reported adverse reactions, which occurred within 2 hours of treatment, were:
- Colic
- Pawing
- Cramping
- Nervousness
- Discomfort
Osphos Inj 60mg/mL, 15 ml ingredients
Osphos intramuscular injection contains the bisphosphonate Clodronic acid
Osphos Inj 60mg/mL, 15 ml overdose: What to do?
Immediately contact your closest emergency animal hospital.
What to know before using Osphos Inj 60mg/mL, 15 ml
Keep away from children.
How is Osphos Inj 60mg/mL, 15 ml sold?
Osphos Inj 60mg/mL is sold in 15ml - single use vial
Manufacturer
Dechra Pharmaceuticals
Tips for using Osphos Inj 60mg/mL, 15 ml
OSPHOS is administered at 1.8 mg/kg by intramuscular injection up to a maximum dose of 900 mg per horse (one vial). Divide the total volume evenly into three separate injection sites. Discard unused vial contents. OSPHOS is provided in a single use vial and does not contain a preservative. If there is no response to initial therapy, the horse should be re-evaluated. For horses that initially respond to OSPHOS but do not maintain their clinical improvement for 6 months, OSPHOS may be re-administered at 3 to 6 month intervals based on recurrence of clinical signs. For horses that respond to OSPHOS and maintain clinical improvement for 6 months, OSPHOS should be re-administered after clinical signs recur.
Overview
OSPHOS is administered at 1.8 mg/kg by intramuscular injection up to a maximum dose of 900 mg per horse (one vial). Divide the total volume evenly into three separate injection sites. Discard unused vial contents. OSPHOS is provided in a single use vial and does not contain a preservative.
If there is no response to initial therapy, the horse should be re-evaluated. For horses that initially respond to OSPHOS but do not maintain their clinical improvement for 6 months, OSPHOS may be re-administered at 3 to 6 month intervals based on recurrence of clinical signs. For horses that respond to OSPHOS and maintain clinical improvement for 6 months, OSPHOS should be re-administered after clinical signs recur
Main Ingredients
Osphos intramuscular injection contains the bisphosphonate Clodronic acid